Partner Article
New partnership sees Ireland's orthopaedic market bring innovative treatment to 100s of patients suffering with knee osteoarthritis
Contura Orthopaedics Ltd has announced an exciting new partnership with Hogan Healthcare, one of Ireland’s leading independent medical device distributors.
Arthrosamid® is a polyacrylamide hydrogel developed by Contura International Ltd. as a long-lasting intra-articular injection approved in Europe for the treatment of knee osteoarthritis (OA).
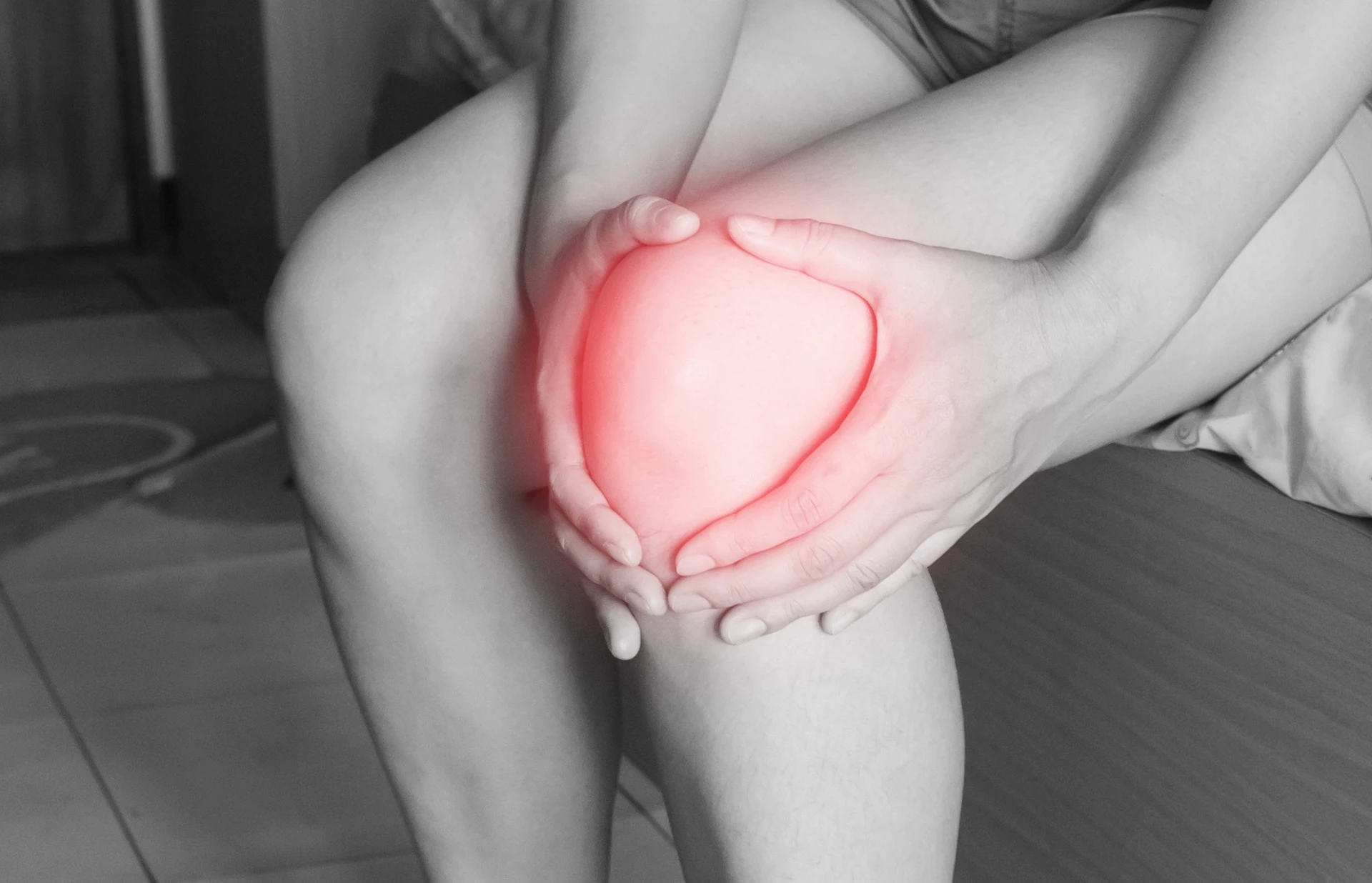
The treatment has the potential to change the present care pathway for managing OA, which affects around one in four adults in Ireland aged over 60 (over 400,000 people) and current treatments are not long lasting or involve invasive knee replacement surgery. Despite a large suffering population, there has been very little innovation in the range of OA treatments on offer to meaningfully help patients – until now.
Consisting of 2.5% cross-linked polyacrylamide and 97.5% non-pyrogenic water, a 6ml dose of Arthrosamid® is injected into the joint space to cushion the knee and relieve pain in one single treatment. Patients can expect to see improved mobility and pain relief within days where the effect is long lasting.
Interestingly, the development of the procedure followed impressive clinical data with Arthramid® Vet, a related product of the company, in the treatment of lameness in horses.
Graham Hogan, Director of Hogan Healthcare, says; “This year marks the 20th anniversary of Hogan Healthcare, and it feels fitting that this new professional association with the team at Contura Orthopaedics Ltd reflects our long-established ethos of working with ‘partners that drive innovation and quality’. What struck us at the very outset of our conversations with Contura Orthopaedics Ltd is that innovation is at the core of this novel treatment. It was something we hadn’t seen before, and we knew we wanted to be ‘leading the charge’ in Ireland as the treatment gains more traction and interest globally. The feedback we’re already hearing from other orthopaedic specialists across Europe is really encouraging, and we’re now so excited to be embarking on a programme of promoting Arthrosamid® to our clinical partners across Ireland.
He adds; “We know there’s a clear need for new treatments which can offer relief to patients suffering from knee pain caused by OA. Waiting lists are at an all time high and many patients – and their physicians – are looking for robust and evidence-based procedures which may help to delay often-inevitable knee surgery. We feel confident and reassured by the research findings already published by the team at Contura and we’re looking forward to furthering data being released – as well as discussing our commitment to the Arthrosamid® procedure at the annual meeting of the Irish Orthopaedic Association in June.”
Several clinical studies have been completed, with further trials ongoing - indeed, new data showing the 2-year results from a propsective open label study (“IDA”) was recently presented at the Orthopaedic Research Society International (OARSI) 2022 World Congress. The results showed that a single injection of the hydrogel continued to be well tolerated and demonstrated clinically relevant and statistically significant effectiveness in reducing pain, at 2 years after treatment.
Rakesh Tailor CEO of Contura International Ltd adds; “We’re absolutely delighted to announce news of our association with Hogan Healthcare as part of our ongoing focus on the European expansion of Arthrosamid®.
“The discomfort caused by knee OA can significantly impact a patient’s quality of life, making everyday activities hugely challenging. For patients not suitable for surgery, or deemed too young for knee replacement surgery, Arthrosamid® represents a game changing option, a safe non-degrading hydrogel in a single injection providing long-acting and sustained pain relief.
“This latest collaboration will enable Contura Orthopaedics Ltd to fulfill our vision of helping many more patients find relief from the pain of knee OA.”
The first procedure has recently been carried out in Ireland took place treating a patient who was likely to be ineligible or knee replacement surgery. The patient will now be followed up closely by the clinician to observe the benefits – and it is hoped this first case study will help to pave the way for further clinics and hospitals across Ireland to adopt the procedure and to begin offering Arthrosamid® as a treatment to other appropriate patients over the coming months.
This was posted in Bdaily's Members' News section by Contura International .








 Putting in the groundwork to boost skills
Putting in the groundwork to boost skills
 £100,000 milestone drives forward STEM work
£100,000 milestone drives forward STEM work
 Restoring confidence for the economic road ahead
Restoring confidence for the economic road ahead
 Ready to scale? Buy-and-build offers opportunity
Ready to scale? Buy-and-build offers opportunity
 When will our regional economy grow?
When will our regional economy grow?
 Creating a thriving North East construction sector
Creating a thriving North East construction sector
 Why investors are still backing the North East
Why investors are still backing the North East
 Time to stop risking Britain’s family businesses
Time to stop risking Britain’s family businesses
 A year of growth, collaboration and impact
A year of growth, collaboration and impact
 2000 reasons for North East business positivity
2000 reasons for North East business positivity
 How to make your growth strategy deliver in 2026
How to make your growth strategy deliver in 2026
 Powering a new wave of regional screen indies
Powering a new wave of regional screen indies